How do voltaic cells work?
1 Answer
A voltaic cell uses oxidation and reduction occurring at two terminals called electrodes to change chemical potential energy into electric potential energy. Operation follows...
Explanation:
In a voltaic cell, we utilize one chemical that has a tendency to oxidize - lose electrons - and a second chemical that will reduce - gain electrons.
In terms of physical requirements, we need two electrodes - solid conductors. One, called the anode will, allow the electrons produced by oxidation to leave the cell, and a second, called the cathode will allow the electrons to re-enter the cell to cause the reduction. Both are immersed in a conducting electrolytic solution.
Any of a large number of materials can serve as these electrodes. Each electrode brings with it a characteristic contribution to the cell voltage known as the half-cell potential.
The voltage of the cell is the difference between these two half-cell potentials.
The diagram below shows the cell consisting of Zn and Cu electrodes, which is commonly the first one students encounter in Chemistry classes. It is typical of the manner in which most cells work.
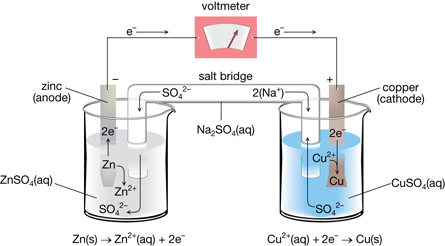
At the anode, zinc metal oxidizes into zinc ions by releasing two electrons. The electrons follow a path through the external wires where a voltmeter measures the energy possessed by the charges. Finally, they arrive at the copper cathode, where they cover the outer surface of the metal.
Here, the electrons combine with copper ions to produce copper metal, and the electrons are removed from the system (that is, they do not flow through the solution back to the anode).
Since positive ions are formed in the left beaker, and removed from the right beaker, it must be necessary for positive ions to flow from left to right. In this setup, this transport of charge is made possible by the "salt bridge". In it, positive ions migrate from left to right, while negative ions move right to left.
The voltage of the cell (at 1 M concentration and 25°C) is 1.10 V. This value is characteristic of the metal and metal ion pairs used in each beaker, or "half-cell". Choice of different metals will cause the voltage to be different.

