Question #dc518
1 Answer
Non-metals transfer their electrons in ionic compounds, forming rigid structures (solids). In molecular compounds, non-metals share their electrons, giving form to many gases and liquids.
Explanation:
In ionic compounds, elements transfer their valence electrons in order to achieve a full electron shell of 8 electrons. Ionic compounds are typically a result of metal to non-metal reactions.
Non-metals act as anions, thus, they are the ones that gain the electrons. This is due to their configuration. Normally having 3 or less electrons away from becoming stable, it's easier for non-metals to gain electrons.
As a result, their electronegativity is much higher than metals.
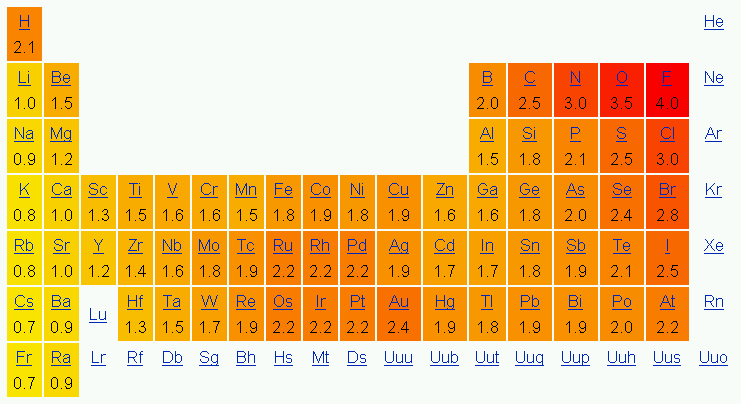
To conclude, the result of ionic bondind forms an attraction-repulsion relationship. This ultimately explains why most ionic compounds are solids.
In covalent compounds, elements share their electrons to achieve a stable composition. Covalent compounds are typically for non-metal to non-metal reactions.
The electrons shared, form an electron pair. Unlike ionic compounds, there is no internal attraction (minuscule), therefore, most molecular compounds are gases and liquids.
Hope this helps anyways :)

