Can the number of nuclear protons change in a chemical reaction? What charged particles are contained in a #"sulfur atom"#, and a #"sulfide ion"#?
3 Answers
Absolutely not.
Explanation:
Sulfur has
Charge is conserved in every chemical reaction. And thus if we have
No.
Explanation:
It's very important to keep in mind that the number of protons never changes when dealing with the ion of a chemical element.
The only thing that changes is the number of electrons that surround the nucleus of the atom.
In your case, sulfur,
This means that a neutral atom of sulfur contains
Now, in order for sulfur to become an anion, it must gain electrons. The number of protons inside its nucleus will always remain constant. Changing the number of protons will change the identity of the element, which is not what we want here.
So, the sulfur anion
You can thus say that the
#16# protons inside its nucleus#-># itm ust contain#16# in order to remain n atom of sulfur#18# electrons surrounding its nucleus
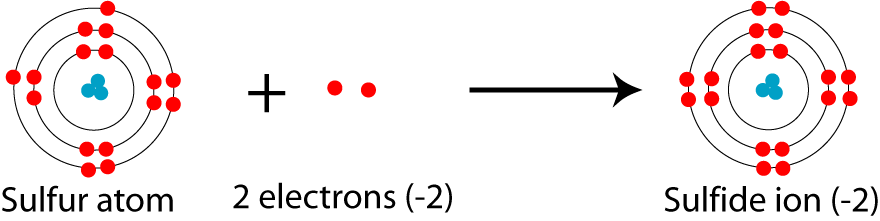
It is a big no.
Explanation:
The proton number does not change in this case. The charge in an atom is simply concerned with the gain or loss of electrons.
When atoms have a negative charge, this means that it has gained some electrons to satisfy its octet.
When sylph is raised to - 2,it means that ityhas gained two electrons.



