What is the O-S-O bond angle in a #SO_3# molecule?
1 Answer
Mar 6, 2017
Explanation:
Given the normal assumptions of VESPER, we have
A Lewis structure of
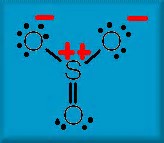
Given the normal assumptions of VESPER, we have
A Lewis structure of
