How do I get the IUPAC names of the compounds below?
1 Answer
Here's what I get.
Explanation:
The general procedure for naming alkanes is:
- Select the longest continuous chain of carbon atoms.
- Number the carbon atoms in that chain from the end that gives the smallest number to the carbon atoms that bear substituents.
- Indicate positions of substituents by numbers.
- Name the compound by prefixing the names of the substituents along with their positions before the name of the parent alkane.
1)
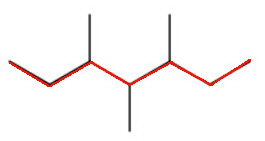
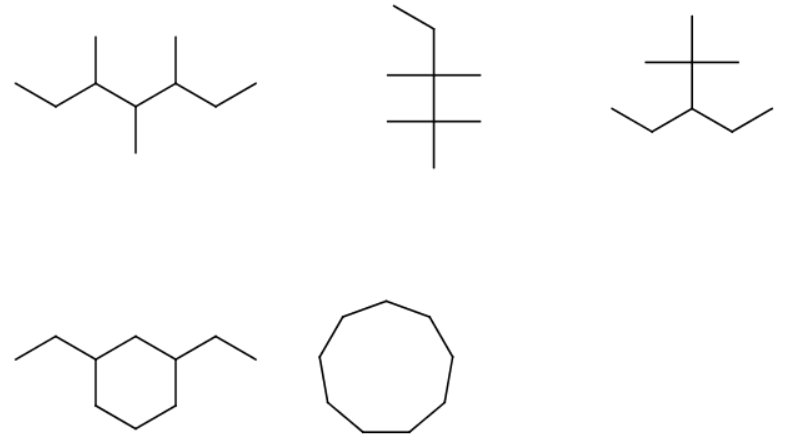
The longest continuous chain contains seven carbon atoms, so the parent alkane is heptane.
When you number the carbon atoms consecutively from one end, you find that there are methyl groups on carbons 3, 4, and 5.
The name is 3,4,5-trimethylheptane.
2)
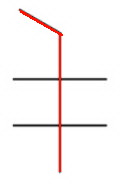
The longest continuous chain contains five carbon atoms (pentane).
There are two methyl groups on carbon 2 and two on carbon 3.
The name is 2,2,3,3-tetramethylpentane.
3)
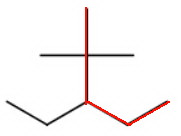
There are several ways to choose the longest continuous chain of five carbon atoms (pentane).
We must pick the chain with the most substituents.
There are two methyl groups on carbon 2 and a ethyl group on carbon 3.
The name of the alkane is then 3-ethyl-2,2-dimethylpentane.
4)
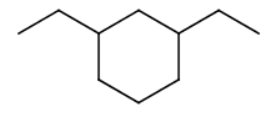
The longest chain/ring is a six-carbon ring (cyclohexane).
There are two ethyl substituents. One of the ring carbons containing a substituent is automatically number 1.
You number the rest of the ring carbons by going around the ring in the direction that gives the lowest numbers to the carbons containing substituents.
The name is 1,3-diethylcyclohexane.
5)
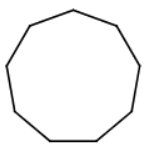
There are nine carbon atoms in a ring.
The name is cyclononane.

