Why is #ZnCl_2# is an acid though it does not have a #H^+# or a #OH^-#?
2 Answers
ZnCl2 is a Lewis acid because of the following reasons
Zn+2 is a Lewis acid
the chlorine does not hydrolyze so the equation would be like this
#["Zn"("H"_ 2"O")_ 6]_ ((aq))^(2+) + "H"_ 2"O"_ ((l)) rightleftharpoons ["Zn"("H"_ 2"O")_ 5("OH")]_ ((aq))^(+) + "H"_ 3"O"_ ((aq))^(+)#
Another way to determine ZnCl2 is acidic is this
6M of ZnCl2 has a pH of 3 - 4
Ksp of ZnCl2 cannot be found on the internet so i think to solve from ZnCl2 solubility in water
Solubility of ZnCl2 =
First convert 100ml to 1000ml or 1litre
4300g must converted to moles
= 31.55mol/1000ml
Whenever ZnCl2 dissolves it dissolves like this
the Ksp expresssion =
The molar ration of 2Cl- to ZnCl2
= 2 : 1
Therefore concentration of 2Cl- is 31.55 * 2 = 63.10mol/L
Ksp =
= 125619.7955
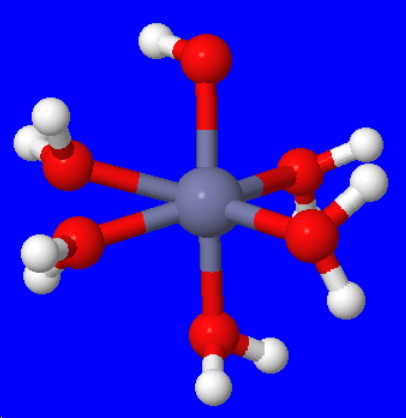
Explanation:
The zinc ion can accept six electron pairs to form a hydrated zinc ion.
The structure of the hydrated ion is
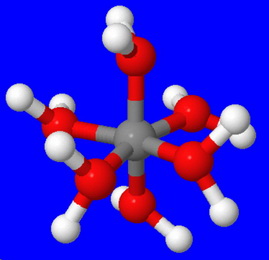
The electronegative
The bonds become weaker, so the water ligands become more acidic.
The aqueous solution forms hydronium ions through reactions like this:
I asked a chemist friend to prepare a 6 mol/L solution of



