Question #78d26
2 Answers
My thoughts are offered below. I hope others will join in...
Explanation:
I'm not quite certain how to frame this question, but here goes!
To my knowledge, there is no such thing as
On that basis, this formula would not represent a molecule that I know of.
If we meant for this to be two water molecules, it should be written
It will be a completely different molecule.
Explanation:
To answer this, you'll need a basic understanding of the Lewis structure and how the Octet Rule works.
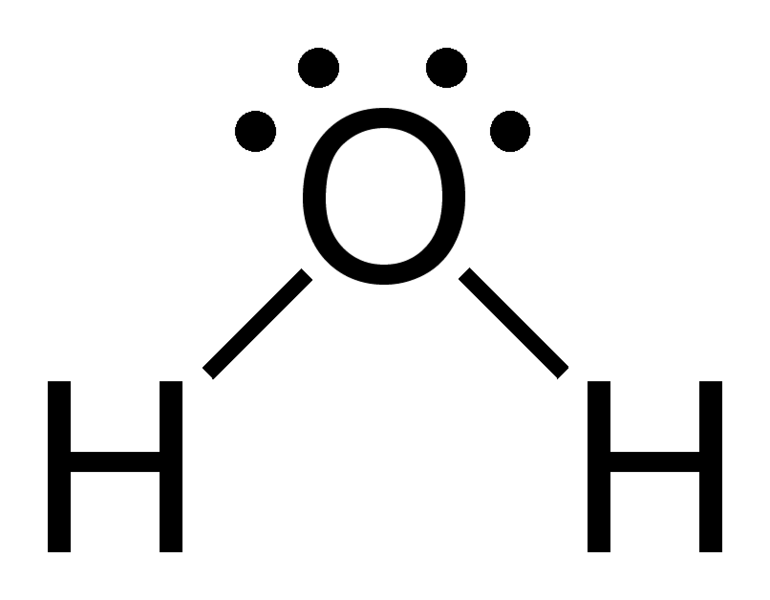
This is how a water molecule (
That is, the
As result of the covalent bonding, the
The formula


