Question #1d40d
1 Answer
Pure water? No. At the pressure required for water to be able to boil at
Explanation:
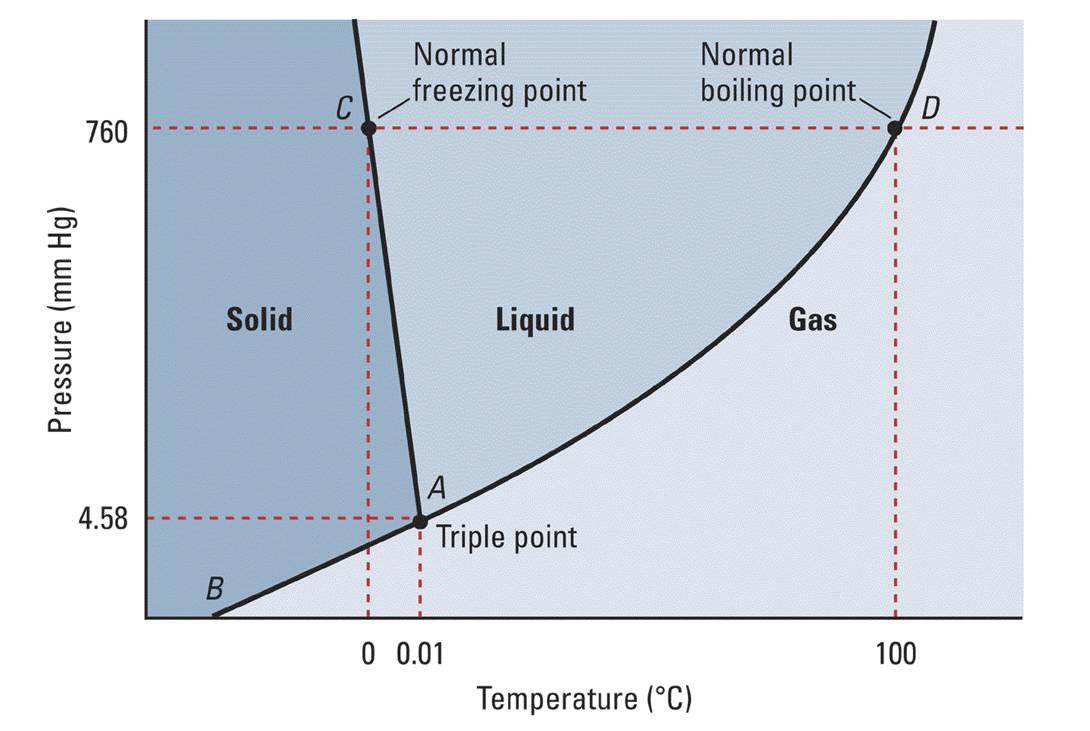
Above is the phase diagram of water. The boiling point at a given pressure is the temperature coordinate of the line between the regions labelled "Liquid" and "Gas" for that pressure coordinate.
As you can see, there is no point on that line where the temperature is


