Catalysts are substances that speed up chemical reaction by doing what?
1 Answer
Apr 17, 2017
By lowering the energy of activation
Explanation:
A catalyst has precisely NO EFFECT on the thermodynamics of a reaction. A catalyst can provide an alternative reaction pathway, and here it acts to alter the
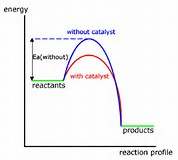
In the diagram the activation enthalpy of the catalysed reaction is LESS than that of the catalysed reaction. Since a greater proportion of reactant molecules will have the requisite activation energy, the catalyzed reaction should be faster. You will likely be asked to reproduced this graph of energy versus reaction profile.

