Question #4670c
1 Answer
Apr 21, 2017
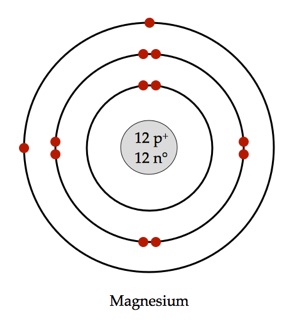
Atomic number for magnesium is
As per Bohr's model the electrons could be arranged inside the atom as per following
- Each shell or energy level can contain only a fixed number of electrons.
- The general formula proposed by Bohr was that the
#n^(th)# shell can contain up to#2(n^2)# electrons. Where#n=1,2,3....# and is the shell number. - The first shell can hold up to two electrons
- The second shell can hold up to eight electrons
- The third shell can hold up to eighteen electrons and so on.
Therefore,
First
Next
Remaining
