Why is the surface tension of water higher than that of ethanol?
1 Answer
Water has greater degrees of hydrogen-bonding in the bulk liquid.
Ethyl alcohol has some hydrogen-bonding, but one side of it is a hydrocarbon (
Hydrogen-bonding is the interaction of a highly-electronegative atom to pull electron density towards it, away from a hydrogen atom connected to another highly-electronegative atom. Here's an example with water:

Here is an example to show that an interaction with a hydrocarbon (or analogously, the hydrocarbon backbone of ethyl alcohol) and water doesn't work.
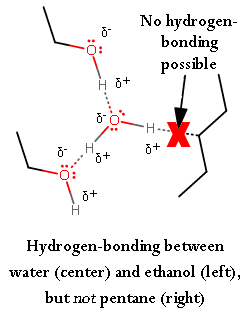
A greater degree of hydrogen-bonding means the molecules are attracted to each other effectively . That is, they stick close together well, and thus, are hard to break apart. As a result, it is more difficult to deform the surface of water than the surface of ethyl alcohol.
Therefore, since water molecules on a liquid surface are harder to push down on the surface tension is higher for water than for ethyl alcohol.

