A sample of silver has a volume of "965 cm"^3". The density of silver is "10.5 g/cm"^3". What is the mass of the silver?
1 Answer
Apr 28, 2017
The mass of the silver is
Explanation:
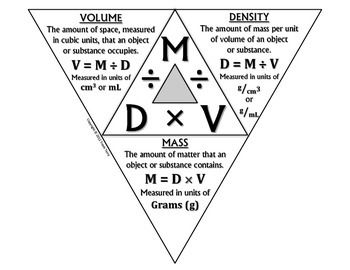
The density triangle below gives definitions of mass, volume and density, and shows how to solve for any of the three variables.