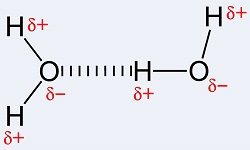
What type of interaction is depicted by the dashed line?
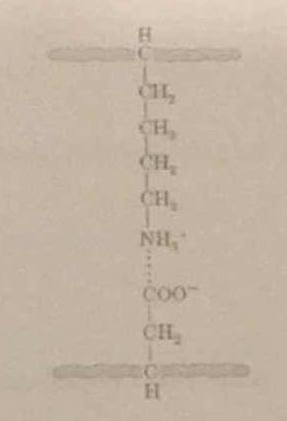
2 Answers
The image is slightly misleading in that the interaction is actually between the second carboxylate oxygen and one of the amine hydrogens.
It is in part a hydrogen-bonding interaction, where the carboxylate oxygen is the hydrogen-bond acceptor, and the amine hydrogen is the donor.
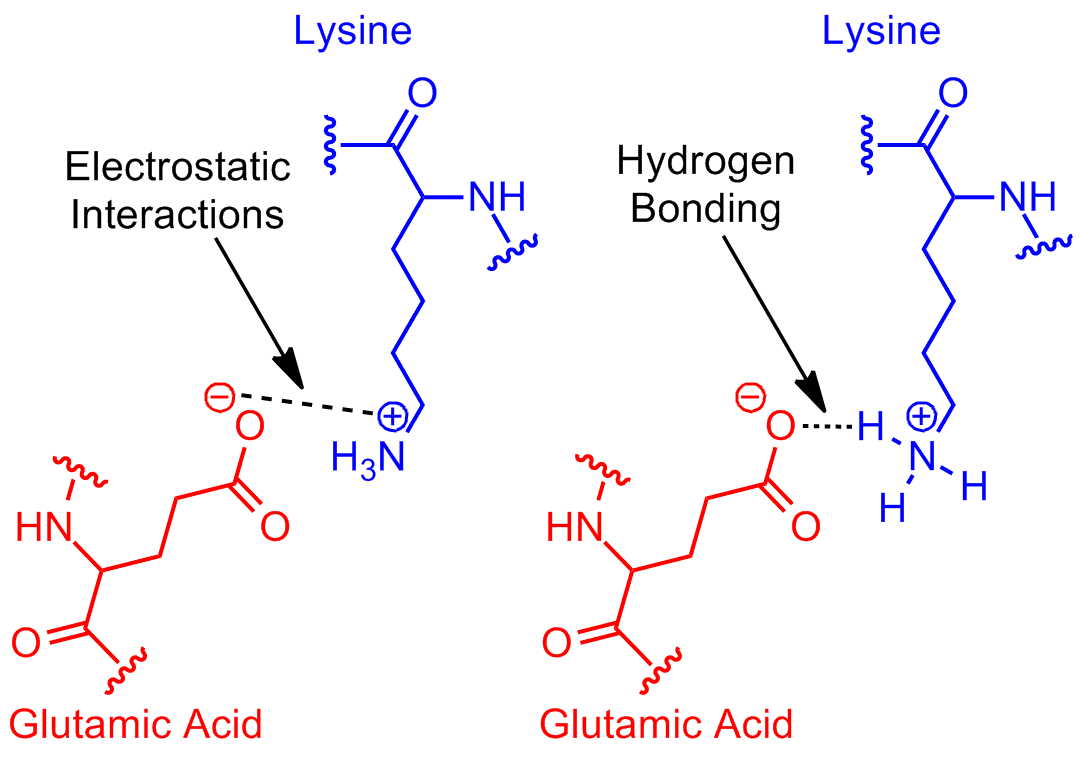
It is also an ion-pairing interaction, between the partially-positive hydrogens as a group and the partially-negative oxygen.
Electrostatic Physical Bond. As shown, most likely 'Hydrogen-Bonding' between electronegative nitrogen and the electropositive carbon center of the carbonyl carbon of the carboxyl group.
Explanation:
There are three types of bonding in nature; physical, chemical and nuclear bonds. Physical bonds are weak electrostatic interactions (i.e., +/- attractions) between elemental structures. Chemical bonds are strong interactions from ion interactions or electron orbital interactions. Nuclear bonds are the strongest bonds involving neutron/proton/electron interactions at the nuclear level.
Within the 'Physical Bonds' classification is the 'Hydrogen-Bonding' which is a special case of dipole-dipole interaction between highly electronegative N, O, or Halogen (F, Cl, Br. I) in which one of the interacting elements is also covalently bonded to a highly electronegative element. By convention, this is typically represented by a dotted line between interacting elements.