With respect to #C-C# bonds, why is a double bond WEAKER than a triple bond?
1 Answer
You mean a
Explanation:
The modern chemical bond.........is CONCEIVED to be a region of high electron density between adjacent atoms such that internuclear repulsion is minimized (due to electrostatic interaction between like charges), and a net attractive force results between the electron cloud, and the positively charged nuclei.
And thus, for single bonds, we say that the electrons are shared between each nuclei:
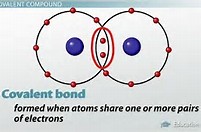
When we introduce multiple bonding, the bonding pairs of electrons are conceived to lie in planes above and below the
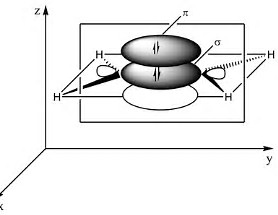
In the representation of the ethylene molecule, there are 4 bonding electrons, and this degree of electron density allows closer approach of the bound carbon atoms. A
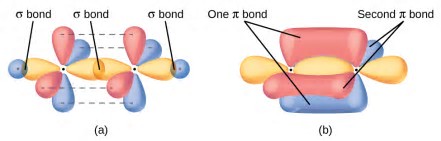
The degree of bonding interaction is not linear. Also see this answer.


