How is an ionic bond formed? How is a covalent bond formed?
1 Answer
Ionic bond is formed by transfer of electrons between metals and non-metals
Covalent bond is formed by sharing electrons between non-metals
Explanation:
Ions that have opposite charges will form an ionic bond in such a way that total charge on the compound is 0.
e.g. Na forms
So the compound formed will be
Covalent bond can be formed between non-metals having like charges e.g Sulphur and oxygen both have -2 charge on their ions, but
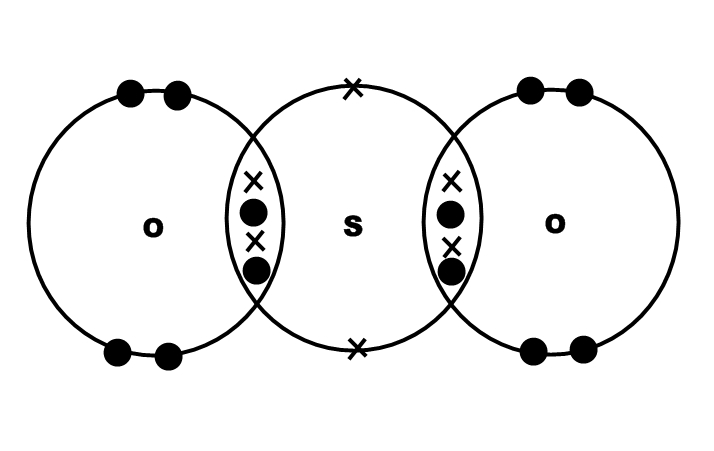
You can find out which type of bond is formed using the difference in electronegativity of the atoms. We have defined the following:
- <0.5 nonpolar bond
- 0.5-2.0 polar bond
- 2.0< ionic bond
The border between these bonds are rough, but give a nice estimate what it probably should be.

