Question #75a19
1 Answer
May 13, 2017
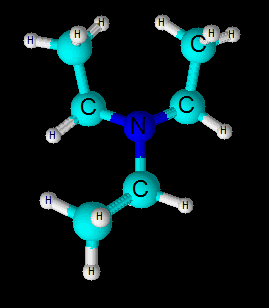
Its molecular geometry is trigonal pyramidal . The central nitrogen atom uses 3
So three bond pairs and one lone pair are tetrahedrally depicted around central N-atom.


