What are the quantum numbers for last electron of cadmium?
1 Answer
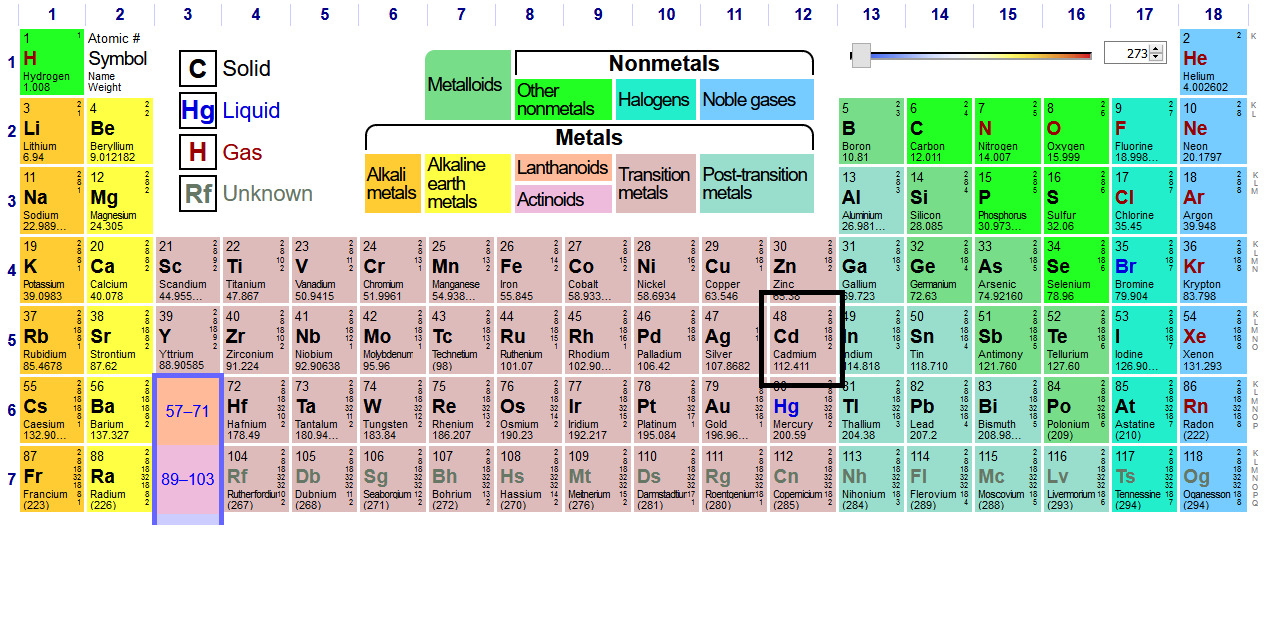
We generally call the main transition metal block (in pink) the
Since cadmium is in the last column of the second-row transition metals (period
In shorthand notation then, we write the electron configuration as:
#[Kr] 4d^10 5s^2# (note that I specifically said
#ns# and#(n-1)d# . That is because#n = 5# for the fifth period, so we have#5s# and#4d# valence orbitals, and not#5d# .)
Since we have no way of proving how exactly the electrons fill the orbitals (we assume!), the "last" electron in cadmium is considered NOT the one that is listed last, but the one that is removed first.
That would be one of the
We do not know which electron will be removed, so we can only say:
#color(blue)((n,l,m_l,m_s) = (5, 0, 0, pm1/2))#

