What is the smallest particle of a compound?
1 Answer
May 19, 2017
See the explanation.
Explanation:
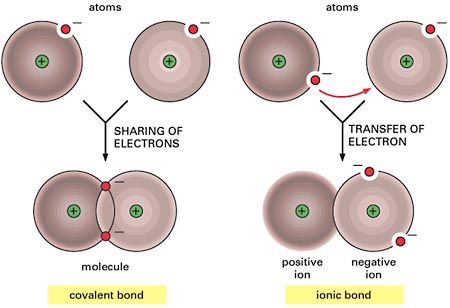
If the compound is covalent (contains only nonmetals), the smallest particle would be a molecule. A molecule is formed when atoms share electrons. The sharing of electrons forms a covalent bond.
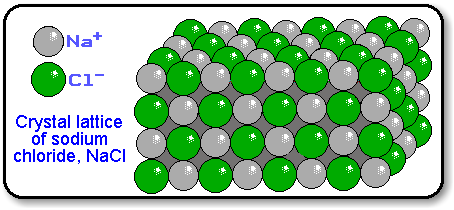
If the compound is ionic (contains a metal cation and a nonmetal anion, or a polyatomic ion), the smallest particle would be a formula unit, which represents the smallest whole number ratio between the elements. This is because ionic compounds form crystal lattices, and there is no discrete smallest particle like a molecule.
 http://www.seplessons.org/node/2241
http://www.seplessons.org/node/2241
 http://schools.bcsd.com/fremont/5th_sci_matter_salt.htm
http://schools.bcsd.com/fremont/5th_sci_matter_salt.htm

