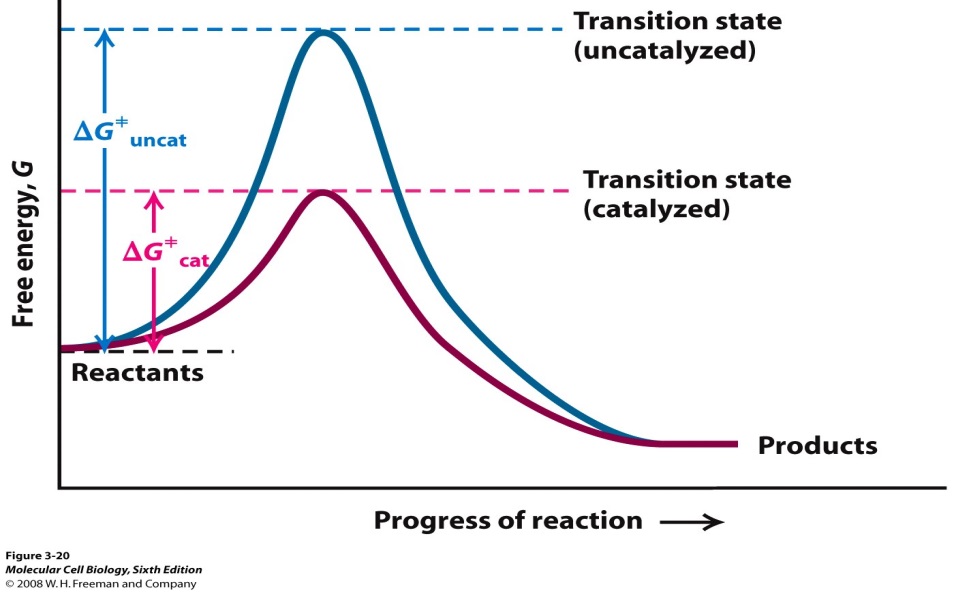
Why does a catalyst cause a reaction to speed up?
1 Answer
May 22, 2017
Catalyst lowers the activation energy
Explanation:
The following Transition State Diagram shows a catalyzed reaction vs an uncatalyzed reaction. In other words, the catalyzed reaction has a lower activation energy 'hill' to climb than the uncatalyzed reaction.