How is the energy level of the products affected by the presence of a catalyst?
1 Answer
May 22, 2017
See below.
Explanation:
A reaction needs a certain amount of energy, called the activation energy, for its reactants to form products.
When a catalyst is added, it provides an alternative pathway for the reactants to form products by lowering the activation energy.
So the products are formed with a lower activation energy.
This does not affect the energy of the products however, as seen in the diagram below for an exothermic reaction.
(energy of products < energy of reactants)
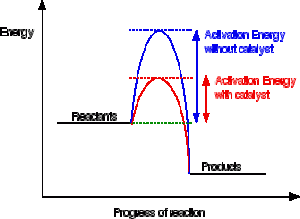
Or an endothermic reaction
(energy of products > energy of reactants)
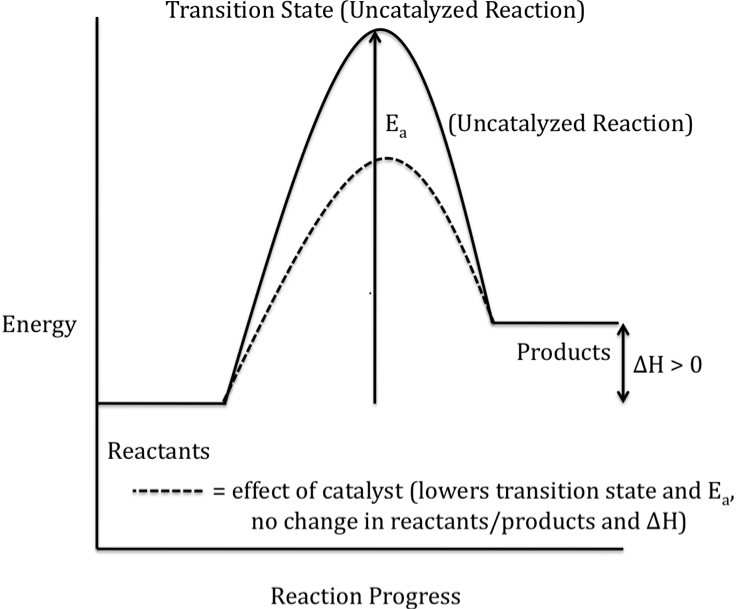
hope this helped!

