Why do we use indicators in acid-base titrations?
1 Answer
May 27, 2017
To signal a stoichiometric endpoint...........
Explanation:
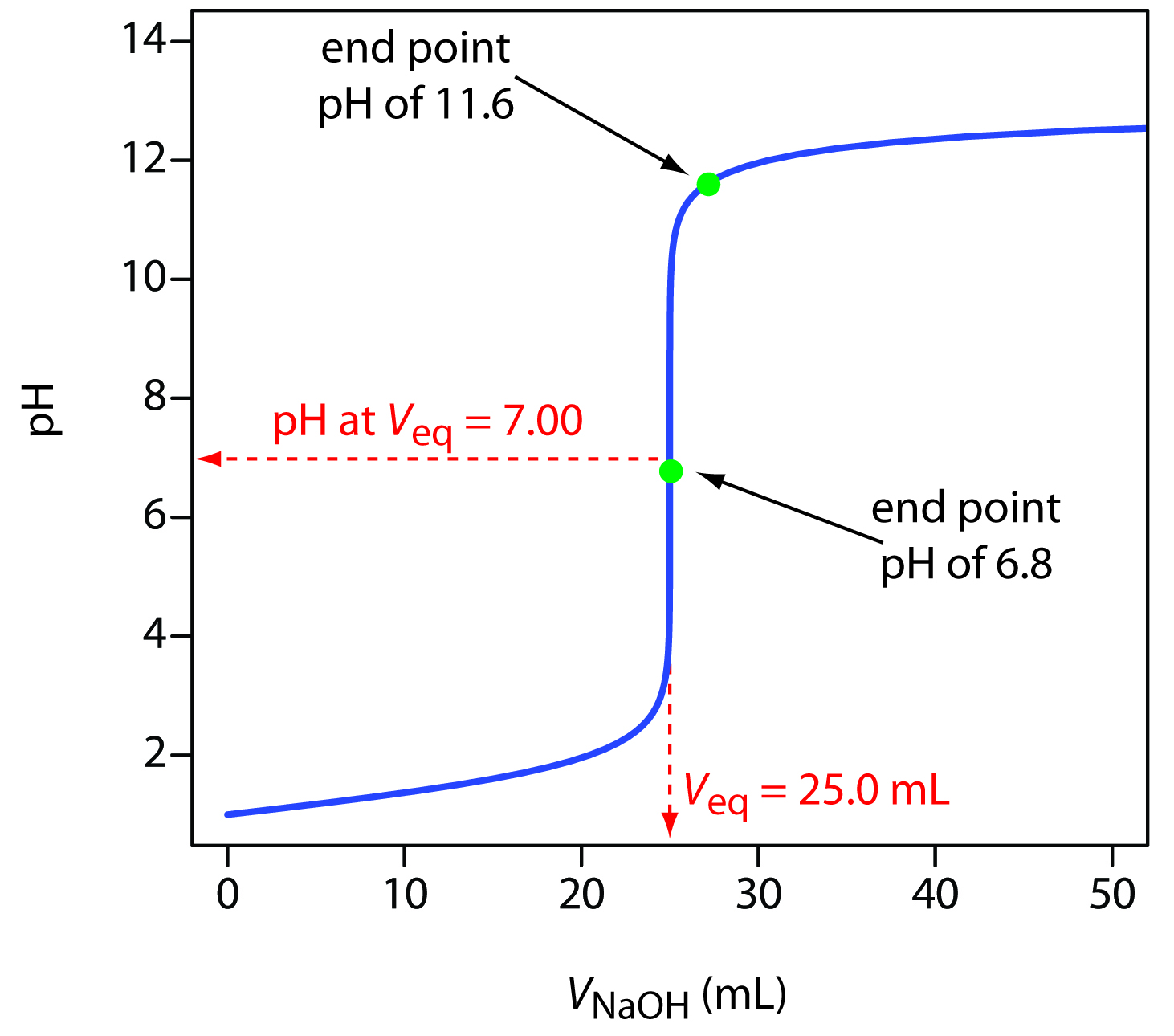
Classically, such indicators were used in an acid-base titration. The precipitous rise in pH during a titration means that a selection of indicators can be used, and