What happens to the kinetic energy of its molecules as ice melts into water?
1 Answer
As ice melts into water, kinetic energy is being added to the particles. This causes them to be 'excited' and they break the bonds that hold them together as a solid, resulting in a change of state: solid -> liquid.
Explanation:
As we may know, the change in state of an object is due to the change in the average kinetic energy of the particles.
This average kinetic energy is proportional to the temperature of the particles.
This is because heat is a form of energy; by adding energy to ice - heat, you "excite" the water molecules, breaking the interactions in the lattice structure and forming weaker, looser hydrogen-bonding interactions.
This causes the ice to melt. This is demonstrated in the image below.
More generally, when you remove energy - the object cools down, the particles move a lot slower. So slow, that they individually attract other molecules more than before, and this results in a physical change that also changes the state.
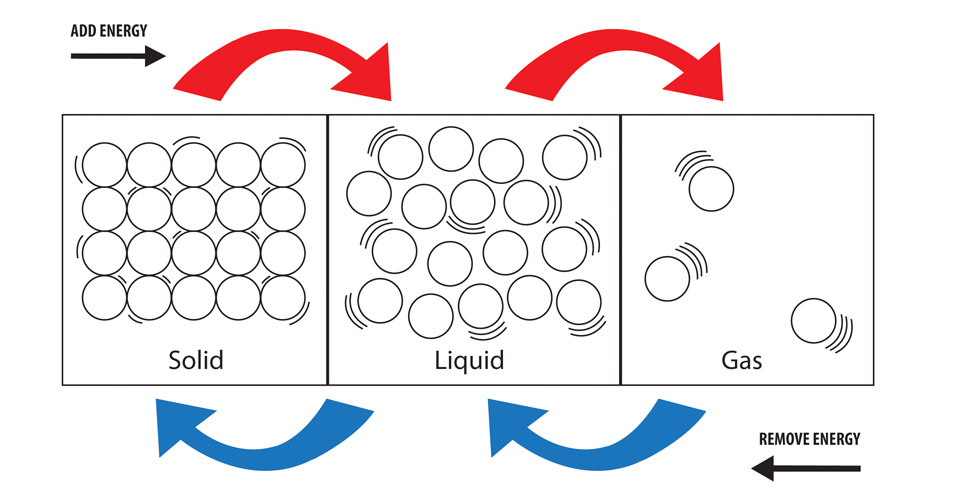
There are other states of matter besides these 3, but these are the most common.
In the case of water melting from ice into water, it is transitioning from a solid to liquid. That means energy is being added in the form of heat energy.
This heat means that the particles gain energy. They then break free of the lattice interactions holding them together (in their solid state). As a result, a change of state from solid to liquid occurs.
Hope this helps :)


