How does a formula unit compare to a crystal lattice for an ionic compound?
1 Answer
Jun 8, 2017
The ratios are the same.
Explanation:
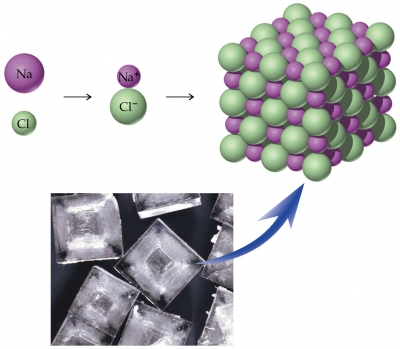
The formula unit of an ionic compound refers to the lowest whole number ratio of ions in the compound, which is the same ratio as the crystal lattice. The formula unit is used because there is no discrete particle like a molecule, because of the crystal lattice.
The diagram below shows how one sodium atom and one chloride atom form oppositely charged ions, forming a formula unit of sodium chloride, which is part of the crystal lattice. Both the formula unit