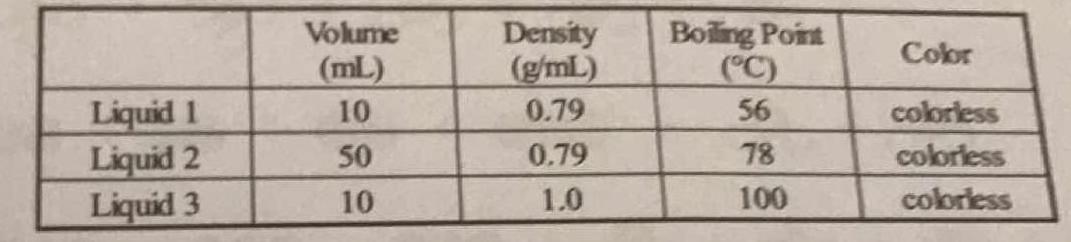
A student determines the volume, density, and boiling point of three colorless liquids and lists them in the table below. Could any of the liquids be the same substance?
2 Answers
Absolutely not.........
Explanation:
A chemical compound has characteristic chemical and physical properties. And we typically use boiling point, and melting point to characterize a material. We got 3 different liquids with DIFFERENT boiling points; and thus they are different materials.
Based on what we know we suspect that
See note in explanation.
Explanation:
Assuming all are at the same temperature and pressure conditions & all are pure condensed phases, then none of the liquids are the same substance. Boiling point and melting points of pure substances are physical properties of the substance. Even if two of the sets had the same measured values for volume, density and boiling point, one should not depend solely on physical properties as identity markers. Such measurements should be supported by spectroscopic tracings that give a more definitive ID to a substance.
One should remember that for a normal assay (i.e., physical and chemical properties) the structure identity is the defining element of substance identity. Once the substance"s identity is known, then physical and chemical properties are associated with the substance.


