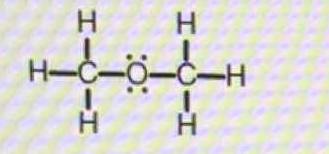
How would you predict the ideal bond angle(s) around each central atom in this molecule?
1 Answer
Jun 13, 2017
Explanation:
Thus the electronic geometry around oxygen is tetrahedral to a first approximation, the actual geometry is somewhat compressed. I urge you to look at the description of

