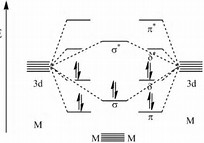
Two atoms are held together in four covalent bonds because of forces between the what?
1 Answer
Jun 17, 2017
There are few examples of a quadruple bond.........
Explanation:
The classic example is
In each case the bond represents a region of electron density between the metal-metal vector, or above the plane of the metal-metal vector, so that internuclear repulsion between the