It dissociates in water until nearly #0%# of it is left. More specifically, its aqueous acid dissociation constant, #K_a#, is (much) larger than #1#.
In general chemistry, i.e. in aqueous media, we define hydronium, #"H"_3"O"^(+)# to have an acid dissociation constant of #1#, or a #"pKa"# of #0#.
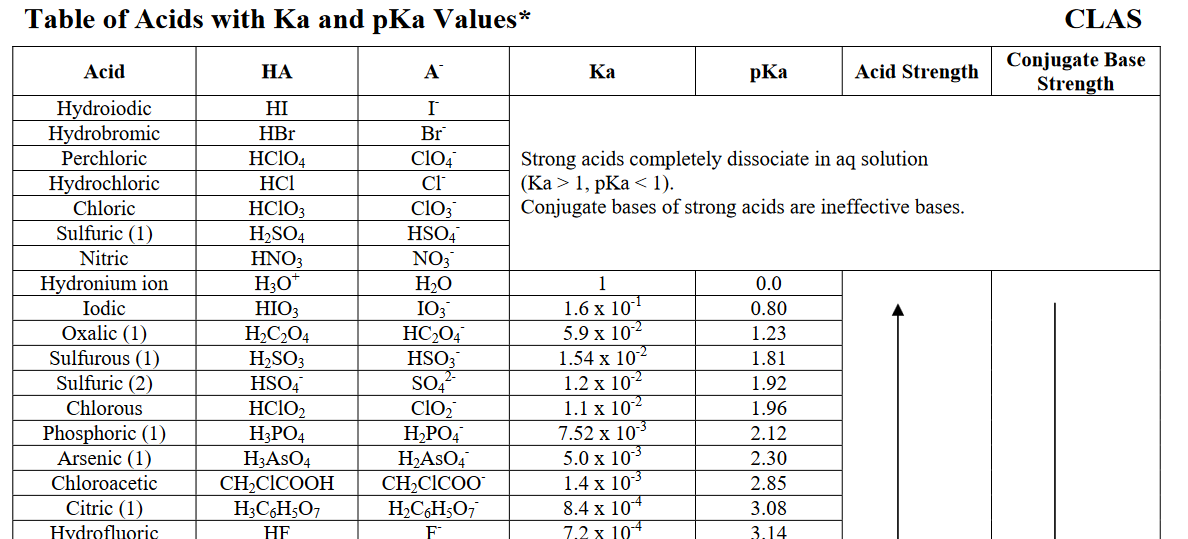
#"HCl"#, relative to this reference, has a #"pKa"# of #-7#, or a #K_a# of #10^(7)#.
Clearly, #10^7# #">>"# #1#, so #"HCl"# is a very strong acid.
On the other hand, something like acetic acid, #"HC"_2"H"_3"O"_2#, is a weak acid, because it has a #K_a < 1#, or it has #"pKa" > 0#:
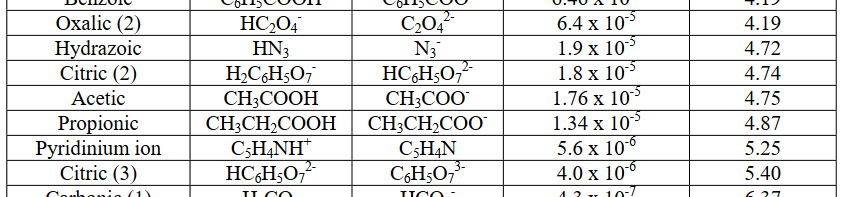
#K_a = 1.76 xx 10^(-5)# #"<<"# #1#
#"pKa" = -log(1.76 xx 10^(-5)) ~~ 4.75 > 0#
Because this hydronium reference is arbitrary and defined for aqueous media, there are #"pKa"# scales in non-aqueous media.

