If a 12.0-g sample of radon-222 decays so that after 19 days only 0.375 g remains, what is the half-life of radon-222?
1 Answer
Jun 26, 2017
The half life is
Explanation:
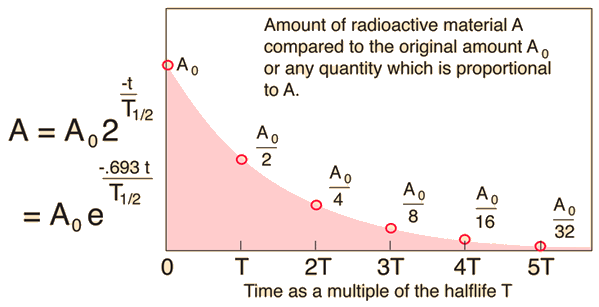
The original mass is
After
The ratio of
That is
The half life is
So,
We can perform the calculation

