To which part of a water molecule would #Li^+# be attracted?
2 Answers
The oxygen part.
Explanation:
A simple way to explain this concept is with something called electronegativity.
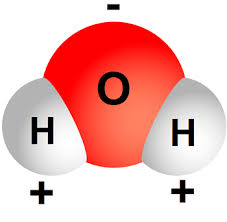
If you were to take a look at the Oxygen atom's electronegativity value, you would be able to tell that it is a very electronegative element. Therefore, it will draw the electrons away from the Hydrogen atoms.
The result will be that the Oxygen will acquire a partial negative charge, and the two Hydrogens will receive a partial positive charge (the sum of the charges would be
Now, lets think about
Therefore, we can say that the
I hope that helps!
Well, which part of the water molecule is most electron-rich?
Explanation:
Clearly we speak of the oxygen centre. When we represent the dissolution of a lithium salt in water, we often write.......
Where


