Why is halogenation anti addition?
1 Answer
Why? Because as far as anyone knows, when...........
Explanation:
........when a halogen adds to a cyclic olefin, it forms a so-called bromonium ion, a three-membered ring......
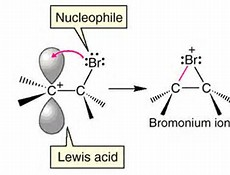
The picture depicts the supposed intermediate after the bromine adds (as an electrophile) to the olefin. We might now invoke a carbocation intermediate, which of course will undergo subsequent reaction with the newly delivered bromide nucleophile....
The formal carbocation MAY be stabilized somewhat by the formation of a bromonium ion, in which the added bromine atom forms a three-membered ring with the potential or conceptual carbocation, thereby somewhat stabilizing it. The newly delivered bromide ion is DIRECTED AWAY from that side of the ring, and thus we get a trans-disubstituted alicycle.
Note that this stereochemical peculiarity is also observed when
Because of the anti-addition,
Anyway, look in your text, because this is an excellent example of a so-called

