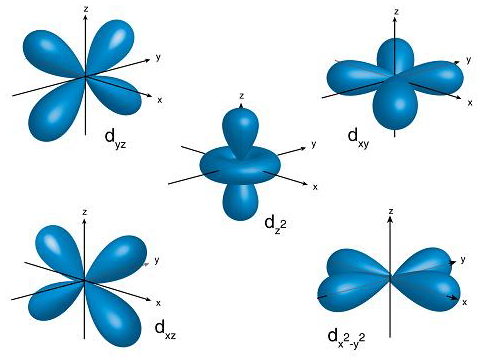
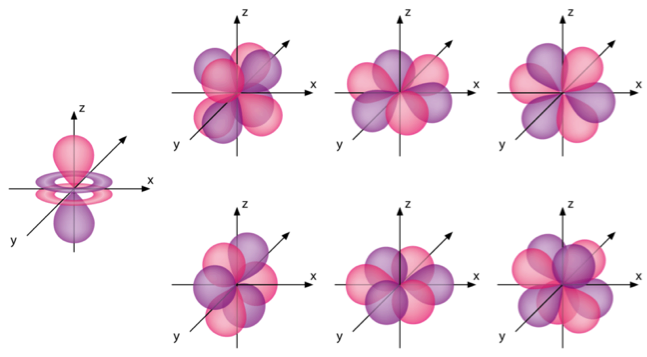How would you describe the shapes and relative energies of the s,p,d, and f atomic orbitals?
1 Answer
Shapes are described below, the energies are on average in increasing order in your question, for the same
Explanation:
Energies of valence orbitals can be found here (Appendix B.9):
http://media.pearsoncmg.com/bc/bc_0media_chem/adv_chem/pdf/11054_appB_ts.pdf
The first five are
What we can say for sure is, though,
#E_(ns) < E_(np) < E_(nd) < E_(nf)# .
The
The