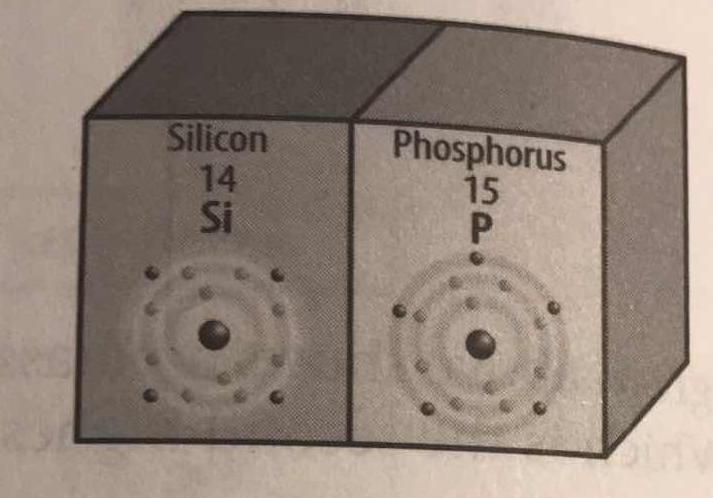
In the diagram, how are valence electrons illustrated? How many valence electrons does each element have?
1 Answer
Well, I would think that the electrons on the outside being DARKER are a good sign that they are important.
And in fact they are; the valence electrons are crucial to effective chemical bonding, and are usually the sole participants in chemical reactions (as opposed to core participation).
Thus, there are
For simplicity, you can find valence electrons for main-group elements (i.e. not transition metals and not
So, in principle (at least for non-transition metals and non-

