With respect to the Periodic Table, how does electronegativity evolve with respect to the individual elements?
1 Answer
Jul 8, 2017
Explanation:
Given that you got
And so the order is
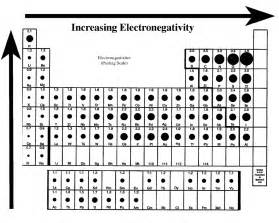
Given that you got
And so the order is
