What phase of #"CO"_2# exists at #-57^@ "C"# and #"2 atm"#?
1 Answer
Jul 9, 2017
This is just asking you to use your finger to trace where you are on the phase diagram. Think back to when you learned about graphs. Treat
Now point to where
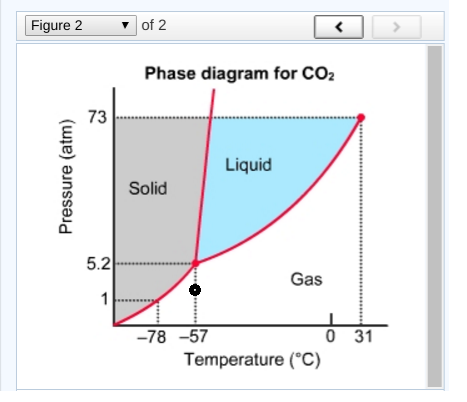
So then, what single phase is here?

