Which of the following is true about ozone?
#A)# The molecule exists with localized bonds, namely one double bond and one single bond.
#B)# The formal charge on the outermost oxygen atoms are #0# and #-1# , respectively.
#C)# The molecule oscillates rapidly between two localized resonance structures.
#D)# Both #"O"-"O"# bonds are equivalent, #1.5# -bonds.
1 Answer
If I chose only one answer, I would choose
Basically, the actual structure of
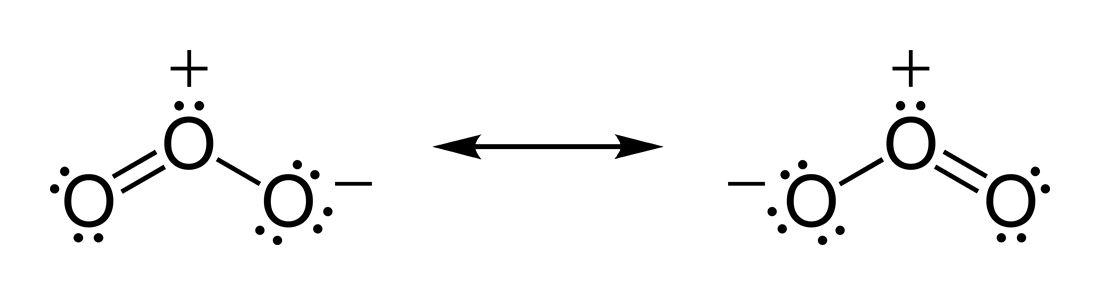
The most correct answer here thus treats both bonds as equivalent, since both resonance structures exist at the same time, but the electrons in the
The electrons in the structure are actually spread throughout the structure, so that both bonds are the same length: the length in between that of a double and a single bond, or a bond order of
To be specific, accepting
But if I chose only one answer, it would be

