What is the difference between nodal surfaces and nodal planes?
1 Answer
Here's my explanation.
Explanation:
A nodal surface is a region of space in which the probability of finding an electron is zero.
There are different types of nodal surfaces.
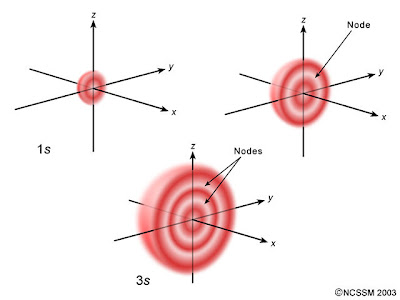
For example. the diagram above compares the electron probability densities for the hydrogen
Note that all three are spherically symmetrical.
However, for all
We could call them spherical nodes or spherical nodal surfaces. They are part of a more general set called the radial nodes.
Also, all
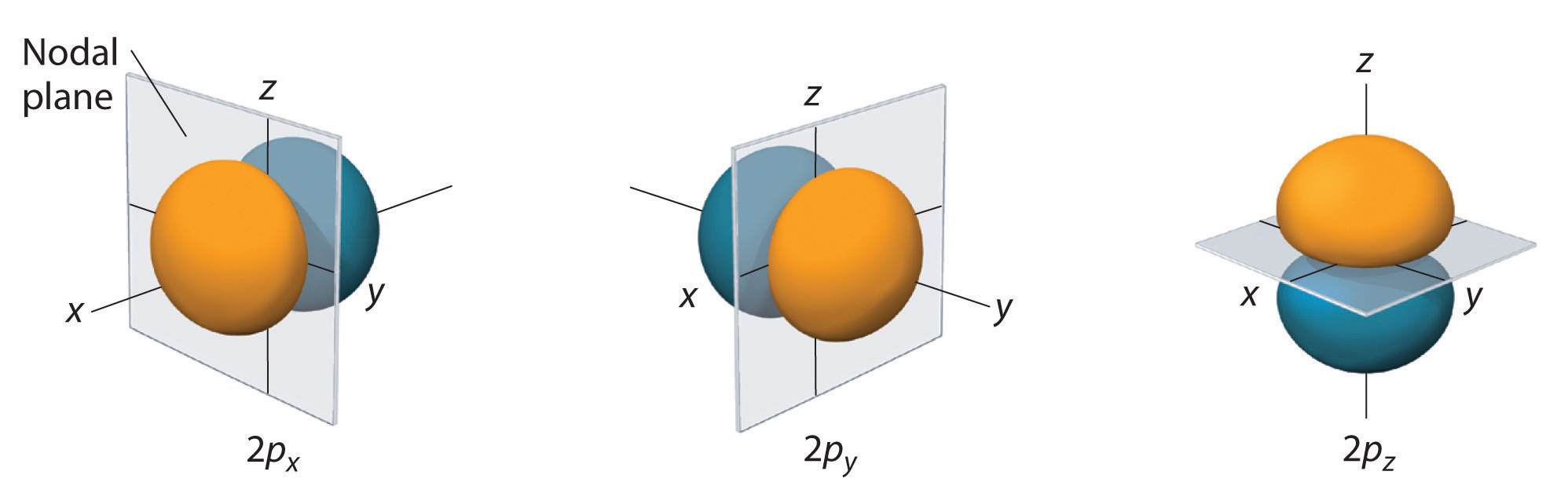
We call these nodal planes or nodal planar surfaces. They are part of a more general set called the angular nodes.
All
For example, a

A



