What is bridge bonding? Does it need backbonding?
1 Answer
Bridge bonding is basically advanced
For example, consider
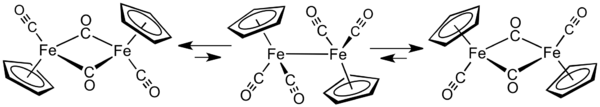
Take the righthand isomer as an example.
The middle
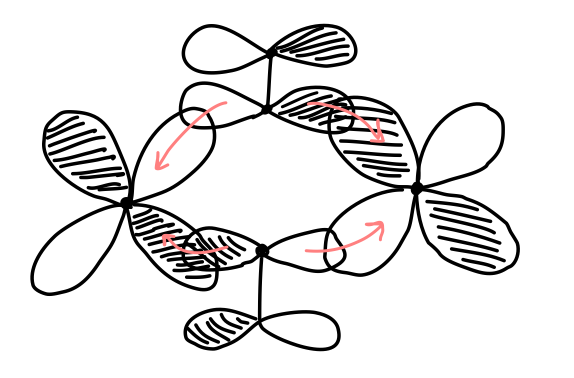
The
- The
#"Fe"# use their#3d_(xy)# atomic orbitals to bond with the#"CO"# #pi_(2px)# molecular orbitals if#x# is horizontal. - The
#"Fe"# use their#3d_(yz)# atomic orbitals to bond with the#"CO"# #pi_(2pz)# molecular orbitals (where#z# is into the screen and#y# is vertical).
The metal
In this case, we cannot see how the backbonding occurs yet; we have to draw the antibonding molecular orbital depiction to see this:
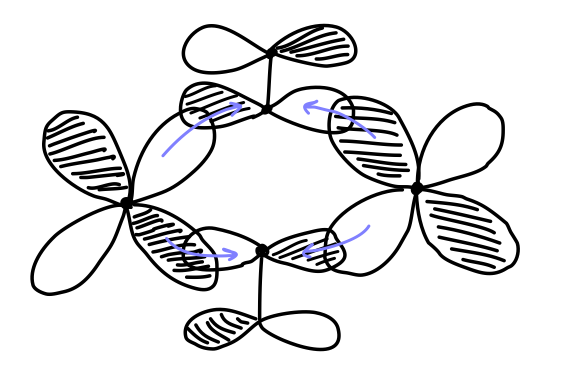
This is possible, since molecular orbitals really have contributions from multiple phase combinations to certain extents. In other words, both of the above depictions occur at the same time, similar to resonance structures.
This weakens the
You can see that both metals could do that at the same time, so a relatively low oxidation state on them favors this (less than
In this case,

