Question #b0a1b
2 Answers
Protons.
Explanation:
The atomic number tells the number of positively charged PROTONS in the nucleus of an atom.
or
The atomic number tells the number of positively charged SUBATOMIC PARTICLE in the nucleus of an atom.
(because positively charged proton can be redundant)
Protons (or just "particles", since it is understandably positively-charged...)
Explanation:
The atomic number (symbol
The number of neutrons and electrons present in the atom can change without changing the identity of the element (respectively, the new particle would be called an
An element can commonly be notated by the form
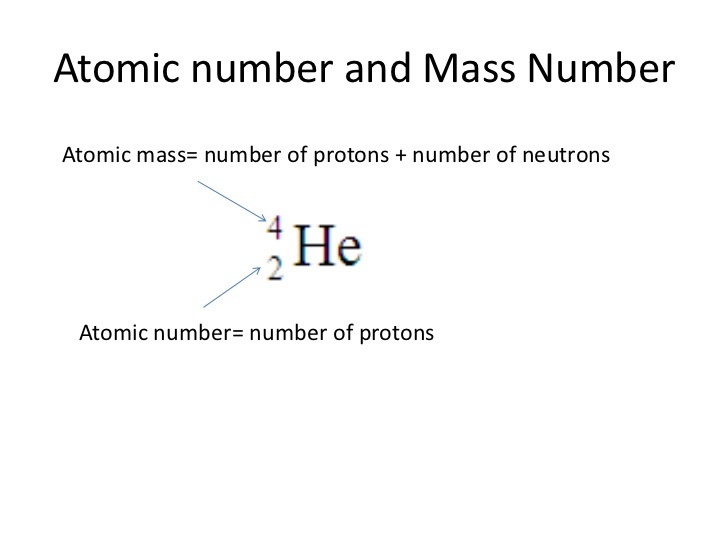 image.slidesharecdn.com
image.slidesharecdn.com
Referring to a periodic table, could you tell me which element has the atomic number 38? or 56? or 101? How many protons do each atom of these elements contain?

