Question #6812b
1 Answer
Here's why that is the case.
Explanation:
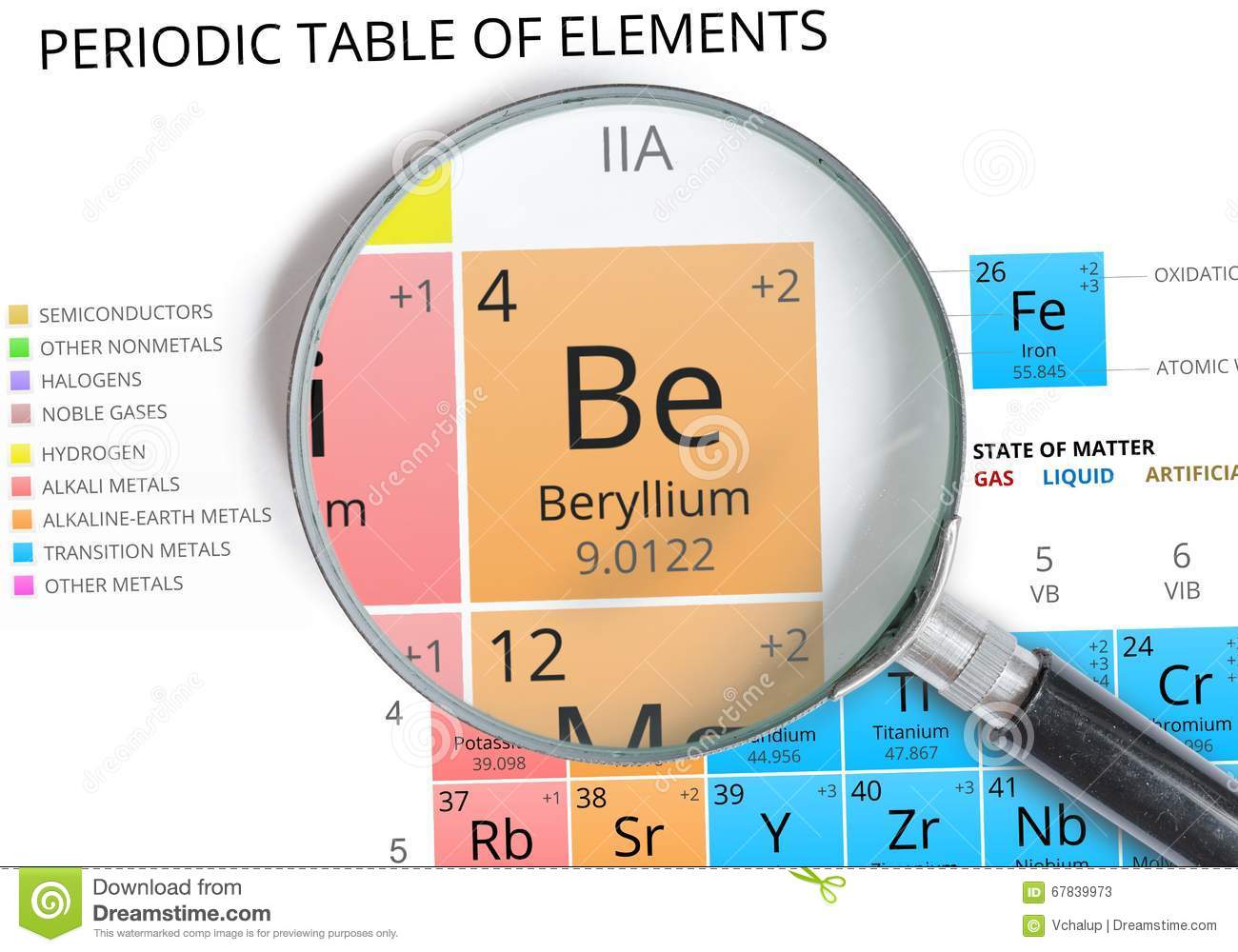
For starters, you know that beryllium is located in period
Now, we can use four quantum numbers to describe the location and spin of an electron inside an atom.
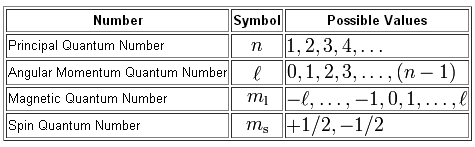
The principal quantum number,
In this case, beryllium is located in period
This means that you have
# n = 2 -># the second energy level
The angular momentum quantum number,
Group
Since you know that you have
#l = 0 -># designates the s subshell#l=1 -># designates the p subshell#l = 2 -># designates the d subshell
#vdots#
and so on, you can say that the fourth electron of beryllium will have
#l = 0 -># the#s# subshell
The magnetic quantum number,
Since the
#m_l = 0 -># the s orbital
Finally, the spin quantum number,
Now, each orbital can hold a maximum of
#m_s = +1/2 -># the electron has spin-up#m_s = -1/2 -># the electron has spin-down
By convention, we take the first electron to be added to an orbital, i.e. the electron added to an empty orbital, to have spin-up and the second electron to be added to the same orbital, i.e. the electron added to the half-filled orbital, to have spin-down.
In your case, the third electron in a beryllium atom is added to the empty
The electron configuration of beryllium shows why that is the case
#"Be: " 1s^2 2s^1 -># the third electron is added to the empty#2s# orbital
#"Be: " 1s^2 2s^2 -># the fourth electron is added to the half-filled#2s# orbital
This means that you have
#m_2 = -1/2#
Therefore, the full set of quantum numbers that we can use to describe the fourth electron in a beryllium atom is
#n = 2, l= 0, m_l = 0, m_2 = -1/2# The electron is located on the second energy level, in the
#2s# subshell, in the#2s# orbital, and has spin-up.

