Question #78d92
1 Answer
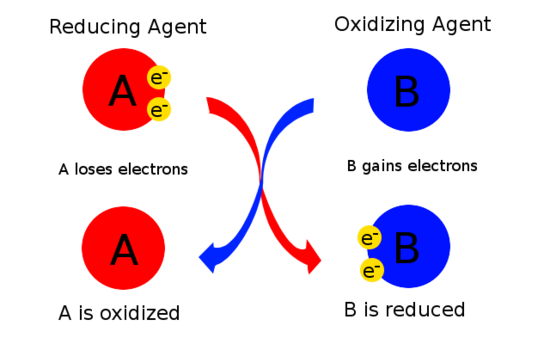
Nitrogen gas is the oxidizing agent and hydrogen gas is the reducing agent.
Explanation:
In this redox reaction, nitrogen gas is acting as the oxidizing agent and hydrogen gas is acting as the reducing agent.
#stackrel(color(blue)(0))("N")_ (2(g)) + 3 stackrel(color(blue)(0))("H")_ (2(g)) -> 2 stackrel(color(blue)(-3))("N") stackrel(color(blue)(+1))("H")_ (3(g))#
As you can see, the oxidation number of nitrogen is going from
On the other hand, the oxidation number of hydrogen goes from
You can thus conclude that nitrogen gas is being reduced to ammonia and hydrogen gas is being oxidized to ammonia.
Consequently, you can say that nitrogen gas acts as the oxidizing agent because it oxidizes hydrogen gas to ammonia while being reduced in the process.
Hydrogen gas acts as the reducing agent because it reduces nitrogen gas to ammonia while being oxidized in the process.