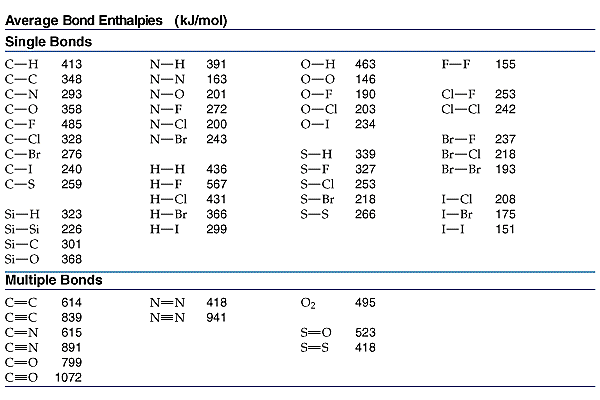
The bond enthalpy, #DeltaH^@#, of the #"C-F"# bond, #"485 kJ/mol"#, is greater than the bond enthalpy of the #"C-I"# bond, #"240 kJ/mol"#. Bond enthalpy is the amount of energy required to break a bond, so the higher the bond enthalpy, the stronger the bond. The other bond found in both #"CH"_3"F"# and #"CH"_3"I"# (iodomethane), is the #"C-H"# bond, which has an enthalpy of #"413 kJ/mol"#. So it is intermediate in strength between the #"C-F"# and #"C-I"# bonds.
 http://www.kentchemistry.com/links/Kinetics/BondEnergy.htm
http://www.kentchemistry.com/links/Kinetics/BondEnergy.htm
https://cbc-wb01x.chemistry.ohio-state.edu/~woodward/ch121/ch8_bondorder.htm
http://www.kentchemistry.com/links/Kinetics/BondEnergy.htm

