How many valence electrons does sulfur possess? And how do we know?
1 Answer
Aug 18, 2017
S does have 6 valence electrons.
Explanation:
As a very general rule- this does not always work, but generally, it does- you can tell how many valence electrons an element has from its position on the periodic table. If you go left to right, counting the number of elements in the s and p blocks up to and including the element, that number is equal to its number of valence electrons.
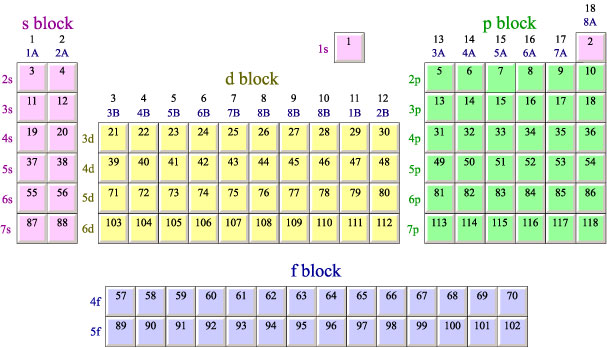
For instance, fluorine has 7 valence electrons. If you count across the table, counting s and p block elements, you get 7. It has 7 valence electrons.


