What are the intermolecular forces in iodine monochloride?
1 Answer
Aug 18, 2017
Explanation:
The
Admittedly, it is not very polar, because
However, there is still a small dipole moment, so the molecule has dipole-dipole forces.
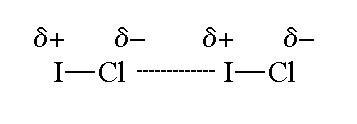
A molecule of
Thus,

