Can someone help me please? This is the last two questions I need help with for tonight. Thank you and have a great night!
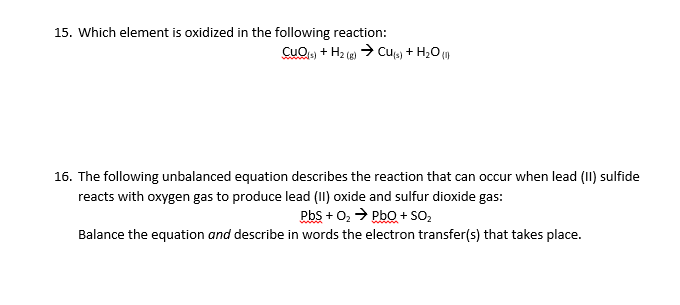
1 Answer
Aug 24, 2017
As regards
Explanation:
So take
But we can simplify the right hand side slightly:
In fact lead ion remains redox inert in this reaction, and remains as
And this is balanced with respect to mass and charge as is absolutely required. Sulfide anion is OXIDIZED to sulfur dioxide, and dioxygen is reduced to oxide.......

