Question #d64a6
1 Answer
Explanation:
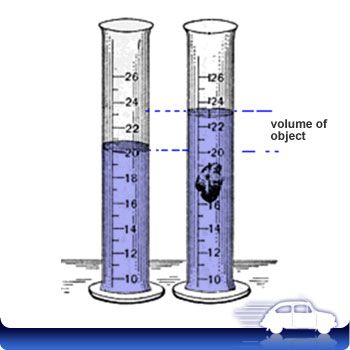
The idea here is that you can determine the volume of the object by looking at the difference between the water levels.
When you add the object to the water, a volume of water that is equal to the volume of the object will be displaced
In your case, the volume of the water displaced by the object will be equal to
#"29.4 mL " - " 25.1 mL = 4.3 mL"#
This means that the volume of the object is equal to
Now, in order to find its density, you must figure out the mass of exactly
Since you know that
#1 color(red)(cancel(color(black)("mL"))) * "5.1 g"/(4.3 color(red)(cancel(color(black)("mL")))) = "1.186 g"#
This means that the density of the object, i.e. its mass per unit of volume, will be equal to
#color(darkgreen)(ul(color(black)("density = 1.2 g mL"^(-1))))#
The answer is rounded to two sig figs.