What effect does temperature have on reaction rate?
1 Answer
Aug 29, 2017
Higher temperature (faster reaction rate), lower temperature (slower reaction rate)
Explanation:
At higher temperature particles get more energy that is greater than or equal to the activation energy (energy required to start the reaction). Therefore, they move faster and collide frequently. As a result, there is more successful collisions per unit time, that means an increased rate of reaction.
When particles are at lower temperature they move slower and don't collide with each other. If they don't collide they don't react. Thus, less successful collisions per unit time and the reaction rate will be slower. Sea diagram below,
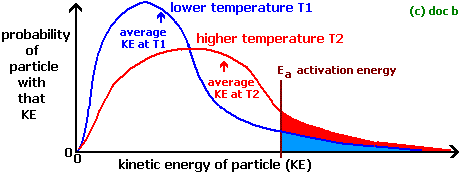
If you want more information on this, go to here

