How do yo write the orbital diagram for hydrogen?
1 Answer
Sep 6, 2017
See below.
Explanation:
Orbital diagrams are useful to show the number of electrons, number of electron shells, number of electron pairs, and electron spin directions in a particular atom/ion. Arrows represent electrons, and their spin is represented by which way they point (up or down). Two electrons can be paired into one shell (one little box) as one orbital. Groups of boxes right next to each other represent groups of orbitals in a shell.
Examples of such diagrams are shown here:
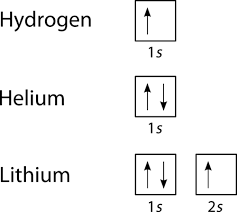
Since Hydrogen only has one electron, it is the simplest one to draw. Only one box (you must label it
I hope that helps!

