Question #43102
1 Answer
Perhaps they do, but they are constrained in different geometries and orientations.....
Explanation:
The modern covalent bond is conceived to be a region of high electron density between 2 positively charged nuclei, such that internuclear repulsion is NEGATED, and a net attractive force between the nuclei and the electron cloud results. The equilibrium distance minimizes internuclear repulsion, and maximizes electron-cloud/nucleus attraction, and this distance is the equilibrium bond length.
A single bond localizes electron density between the nuclei.
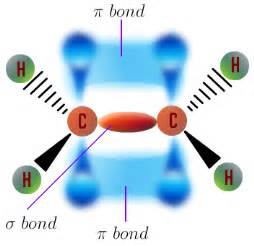 chemistry.tutorvista.com
chemistry.tutorvista.com
And a double bond localizes electron density above and below the plane of the atom-atom vector, and given Cartesian geometry, 2 axes are possible for this overlap, as the diagram shows.....
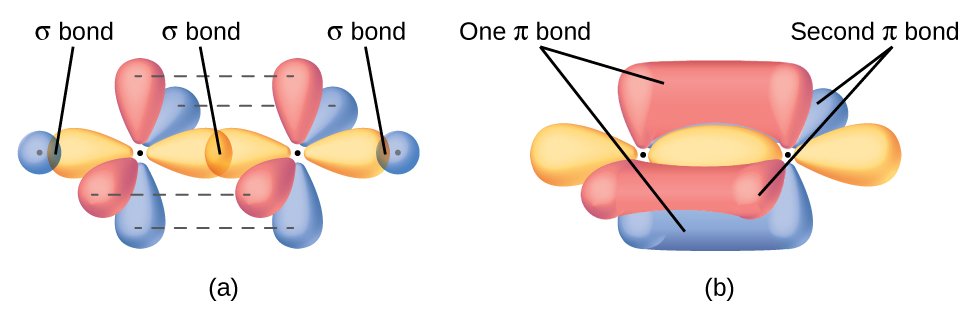 www.socratic.org
www.socratic.org
For the 2
Quadruple bonding, so-called

