The least electronegative group is the...?
2 Answers
..is the Group 1, i.e. the alkali metals.......
Explanation:
Electronegativity is conceived to be the ability of an atom in a chemical bond to polarize electron density towards itself. There are various scales, of which the Pauling scale was the earliest, and still is very widely used.
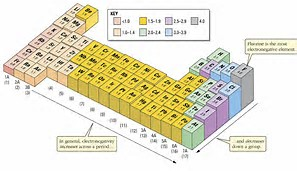
You cannot see the values on the graph (or at least I cannot without my spex). but clearly the alkali metals are the LEAST ELECTRONEGATIVE. For a given Period, these have the LEAST nuclear charge, and also the least shielding by other electrons (only the one electron is present in their valence shells.
The least electro negative group is VIII the inert gases.
Explanation:
The inert gases have an effective electro negativity of zero.
Because the inert gases are stable there is no attraction for more electrons

