Question #f0ce3
1 Answer
Grab a Periodic Table!
Explanation:
The number of protons located inside the nucleus of an atom is given by the atomic number,
For a neutral atom, the number of protons that are located inside the nucleus is equal to the number of electrons that surround the nucleus.
This implies that for any neutral atom, the atomic number tells you two things
- the number of protons in the nucleus
- the number of electrons outside the nucleus
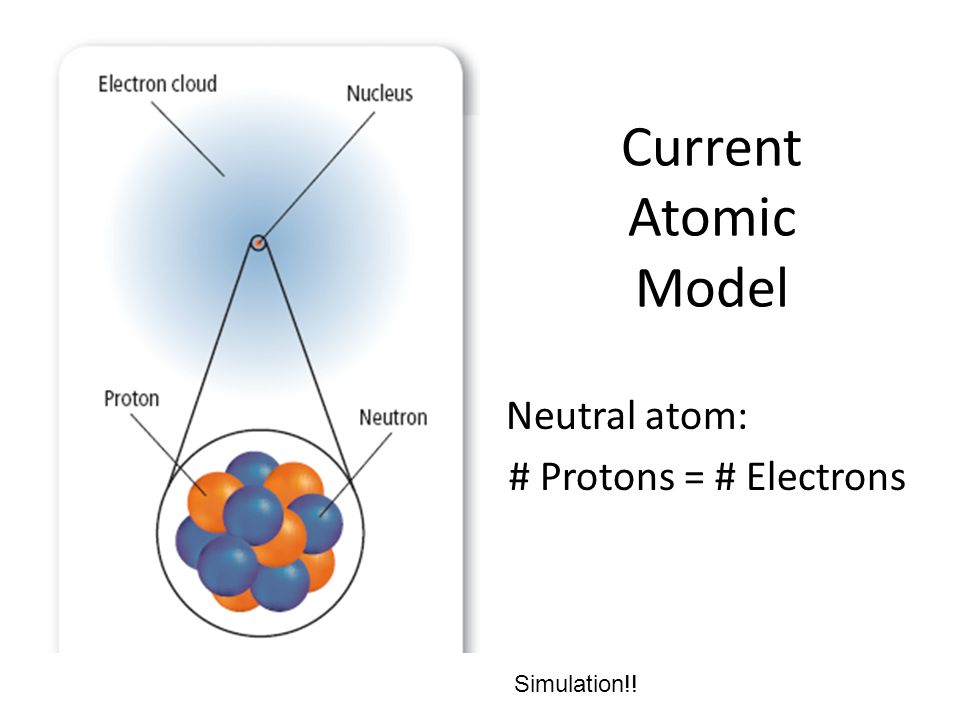
So all you have to do in order to find the number of protons and the number of electrons in a neutral atom is pick up a Periodic Table and look for the atomic number of the element.
If you're dealing with an ion, then you have to consider the overall charge.
The charge of an ion is given by
#color(blue)(ul(color(black)("net charge" = "no. of protons " - " no. of electrons")))#
The number of protons is still given by the atomic number.
So, for example, if you're dealing with a sodium cation,
#Z = 11#
The sodium carries a
#1+ = 11 - "no. of electrons"#
This means that a sodium cation has
#"no. of electrons" = 11 - 1 = 10#
A neutral sodium atom has

